The controversy over genetically modified products seems never to have ceased. The focus is mainly on food safety and environmental threats. However, when genetic technology attempts to introduce human genes into plants or animals, the controversy is no longer confined to science alone. Ethical discussions were also introduced. If foods and medicines containing human genes are available, are we taking medicines or eating people? A newly developed anti-dysenteric protein powder in the United States has been pushed into the cusp of the storm.
Introducing human genes into rice It has been a long time since the GM rice event that occurred in the United States was not the first report that introduced human genes into rice. On April 24 last year, the British "Ismail" also reported a similar incident in Japan. Japanese researchers inserted genes from the human liver into rice genes to enable rice to digest pesticides and industrially produced chemicals. At the time, the study raised the controversy surrounding genetically modified foods to a new level. Environmentalists even thought that eating foods containing human genes was somewhat similar to human consumption.
The newspaper also interviewed Fang Zhouzi and relevant staff of Greenpeace, an environmental protection organization that has always been concerned about the safety of genetically modified products (for details, please refer to “Beijing Science and Technology Newsâ€, May 18, 2005, A2 Edition, “Human Genes and Rice Societyâ€). Whether it causes people to eat people"). In an interview, Fang clearly stated that it is ridiculous to think that eating genetically engineered rice is “eat peopleâ€. The introduction of human genes into rice is intended only to take advantage of the special functions of certain human genes, so that new good traits appear in rice, and there is virtually no difference with ordinary rice.
Greenpeace’s Shi Pengxiang has consistently maintained a green and peaceful approach to GM products with respect to the availability of human genetic rice. He believes that the release of genetically modified rice poses a potential threat to the environment, and that rice, as a staple food for humans, should not be released in the GM rice at the research stage.
Is the drug extracted from human genetic rice safe and effective?
Unlike Japan's GM rice, the Ventriola GM rice is not directly used as a staple food for humans. Instead, it uses two kinds of special proteins to extract anti-diarrhea drugs. However, some farmers and environmentalists have raised their own concerns. They believe that this “medicinal crop†mixed with traditional crops will make them unsafe to eat. Hopesand, a member of the ETC Group, a nonprofit environmental organization in Cal Por, North Carolina, said: "This medicinal product will be grown in outdoor growing food crops. The probability of this product contaminating traditional crops It's very big. It's a risky thing."
Scott Dieter, president of Ventria, insists that rice is propagated through self-pollination, so that these genetically modified transgenic rice cannot actually be occasionally crossed with traditional crops. Moreover, Ventria also stated that the chances of the GM rice they produce eventually entering the food supply market are minimal, because before they are shipped out, they will crush the rice and extract the protein from it. "We use a relatively strict system to closely monitor the flow of GM rice," Dieter said.
At the same time, Ventria has filed an application with the US Food and Drug Administration (FDA) and hopes that this anti-diarrhea protein powder can be approved as a “pharmaceutical food†rather than a drug. This means that Ventriola does not need to carry out long and costly human tests.
In early May, a Peruvian scientist sponsored by the Venturias company provided a large amount of scientific data to the American Academy of Pediatricians Association meeting in San Francisco. These data show that in Peru, if children with severe illnesses in the dysentery regimen contain the antidiarrheal protein powder, their recovery time is 3.67 days, and children who do not take this antidiarrheal protein powder need 5.21 days. Rehabilitation.
Dieter said he hopes to pass the approval of the Food and Drug Administration this year. Dieter predicts that annual sales of anti-diarrhea protein powder in the United States will reach 100 million U.S. dollars, and sales in overseas markets will reach 500 million U.S. dollars, especially in developing countries, because dysentery is the number one killer of children under five in these countries. . According to the World Health Organization report, nearly 2 million children die each year from diarrhea.
How to dispel people's doubts about "taking medicine or eating people?"
Although the development unit is trying to minimize the negative impact of “human genetic rice,†it still cannot dispel people’s doubts about “taking medicine or eating peopleâ€. In spite of boycotts and protests, Ventriola was not discouraged and eventually owned a field near Greenville, North Carolina. In March this year, the company was approved by the United States Department of Agriculture to expand its experimental plot area from 70 acres to 335 acres. Ventria also hopes to receive approval from relevant authorities this year for the marketing of its antidiarrheal protein powder.
Ventria hopes to add this anti-diarrhea protein powder to existing baby products. The United States has not yet stipulated that the food label must contain the genetically modified ingredients it contains. But now, it seems that dispelling the consumer's concerns in the short term and the management's concerns about using genetically modified products to feed babies are still unrealistic. In particular, the obstruction of the American Association of Commodity Producers has made this prospect even more difficult.
- Text / Reporter Liu Hui - Compiler / Yang Xiaowen News Background Human genes introduced into rice for anti-diarrhea medicine According to the Associated Press, Ventriola Biotech is currently developing a drug against diarrhea, but this The drugs have not yet come out, they encountered boycotts and protests because the company introduced human genes into rice through genetic means. Environmental organizations, relevant interest groups, and thousands of farmers across the United States have successfully “pulled out†Ventria's rice testing sites from two states. Critics constantly complain that Ventriola uses most untested genetically modified technologies to grow rice, threatening the safety of traditional crops.
The “biopharmaceutical†technology used by Ventria is essentially aimed at transplanting human genes into crops, thereby forming proteins that can be extracted into drugs. This technology is currently the most controversial part of agricultural biotechnology.
According to the report, Ventria's GM rice can produce two proteins that can be found in breast milk, saliva, and tears. These two proteins help the body's hydration response, thereby alleviating rickets and shortening duration.
Experts linking genetically modified technology in the field of biomedicine is widely used Professor Wang Guoying, a professor of biochemistry and molecular biology at China Agricultural University, has been engaged in research on genetically modified crops for many years. He believes that the introduction of human genes into rice is entirely possible from a biotechnology perspective. What is new.
Regardless of human genes, animal genes, or plant genes, if they are only one nucleotide fragment for a single gene, genetic engineering technology is now more developed because it can transfer human genes, microbial genes, and animal genes. Go to the plant. The plant itself can produce a wide variety of plant proteins. After inserting animal genes or human genes, many proteins can be translated according to their own codes, and no animal genes or human genes can be identified at all.
In fact, in the field of biomedicine, the application of genetic engineering technology is very common. For example, medicinal insulin and interferon are genetically engineered products. Since there are few sources of insulin extracted from the human body, they are later extracted from animals, but the price is very high. Genetic engineering technology is now used to transfer insulin to micro-organisms. Through fermentation, micro-organisms produce insulin, which is actually a genetically modified technology.
The cross arm and control cabinet of the Tower Crane adopt the imported high-strength aluminum alloy ofpatent technology for the one off extrusion molding, Icu Bridge and the surface undergoes the primary oxidationtreatment.
Pneumatic brake plus mechanical friction damping brake
The imported electrical machine is adopted, and the electric perpendicular moving up and down.The gas pipeline, power supply and computer communication line are separately arranged withoutinterference.The imported (GENTEC) brand German standard gas terminal (over 20,000 of inserting and pulling out) is adopted.The ICU Bridge with high bearing capacity with suspension the cavity mirror car system.The horizontal rotation function can accurately position without excursion.
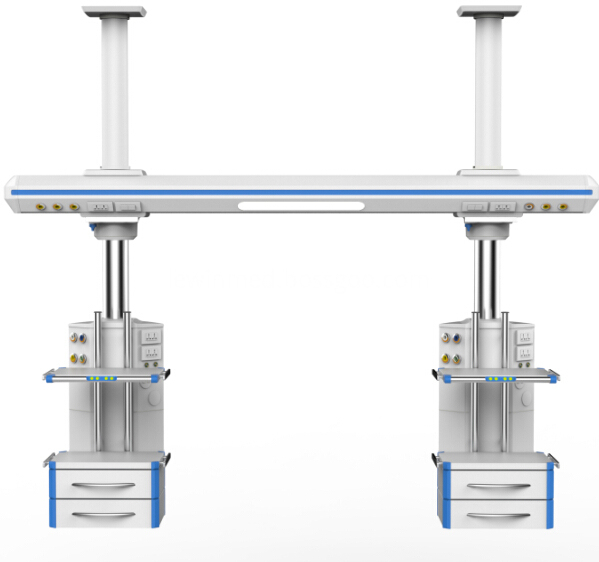
ICU Bridge
Icu Bridge,Icu Bridge Pendant,Portable Supply Unit,Tower Crane
Shandong Lewin Medical Equipment Co., Ltd. , http://www.lewinmed.com