Cory J. Gerdts, a, b, c Glenn L. Stahl, a Alberto Napuli, d, e Bart Staker, a, d Jan Abendroth, a, d Thomas E. Edwards, a, d Peter Myler, d, f Wesley Van Voorhis, e Peter Nollertb and Lance J. Stewarta, b, c, d*
MPCS uses droplet-based microfluidic chip crystallization technology to produce nanoliter volumes of protein crystals for X-ray diffraction. In this study, 29 (93%) of the crystals obtained by the traditional meteorological diffusion test were successfully crystallized by the chemical gradient optimization experiment using MPCS technology. In total, the number of crystals collected by the 120 protein/precipitant combination in the meteorological diffusion experiment was 90 (75%) successfully crystallized using the MPCS technique. Many of the crystals obtained have high quality X-ray diffraction data, and six new protein structures harvested from MPCS CrystalCards are reported.
introduction
The focus of improving the success rate of protein crystallization is the continued research and development of new technologies (Fox et al., 2008; Ng, Clark et al., 2008; Ng, Stevens & Kuhn, 2008; Li et al., 2009, 2010; Hansen Et al., 2002; Cherezov et al., 2008, 2009; Dhouib et al., 2009; Sauter et al., 2007; Hansen & Quake, 2003). Protein crystals are often difficult to obtain, so crystallizers try to use new crystallization techniques, even if there is only a small increase in crystallization success rate. However, if the value of the crystallizer can be confirmed, this new technology will be widely accepted. In this field, the value of crystallizers is measured by how to produce high quality crystals and crystal structures for diffraction with a limited amount of protein.
ATCG3D (The Accelerated Technologies Center for Gene to 3D Structures) has developed MPCS, a droplet-based microfluidic chip protein crystallization technology that can quickly and easily create hundreds of batch-under-oil- Style crystallization (Gerdts et al., 2008). MPCS technology is unique because it produces hundreds of nanoliter volumes (10-20 nL) of crystals, each containing different chemical compositions (Gerdts et al., 2006; Zheng et al., 2003, 2005). Since the result is on the chip, a series of droplet concentration gradients can be well controlled, which can effectively optimize protein crystals. In addition, the peel-apart CrystalCards are part of the MPCS and are used primarily for simple crystallization extraction prior to diffraction experiments. This technique, combined with these advantages, enables detailed optimization of the number of crystal samples, and the resulting crystals can be directly used for downstream diffraction experiments. In this study, we tested the ability of MPCS technology to optimize crystals using 29 different soluble proteins provided by SSGCID.
2. Research workflow
SSGCID is one of two centers funded by the National Institute of Immunization and Infectious Diseases (NIAID) and is a consortium of four Northwest Pacific institutions (Seattle BioMed, Emerald, University of Washington and Battelle). The main task of SSGCID is to determine 75 each year. - 100 new protein structures for the study of AC targets in the NIAID category as well as emerging and five-year re-emergence of infectious diseases. In this study, SSGCID protein was used to detect the ability of MPCS to rapidly optimize protein crystallization conditions using the traditional meteorological diffusion method in hanging drop experiments. Figure 1 shows the research workflow. The purified SSGCID protein was screened using the hanging drop method in meteorological diffusion method and compared to a series of common crystallization screens (Wizard I, Wizard II, Wizard III, JCSG+ and Precipitant Synergy from Emerald BioSystems, and Crystal Screen HT and Index HT from Hampton). Research). Removal without crystallization at first - consistent with the workflow for high-throughput SSGCID structure determination. If the first screened single crystal can be used for X-ray diffraction, the diffraction quality should be first tested before using MPCS optimization (this is done to avoid any improvement in crystal optimization). The initial microcrystals or single crystals produced by the protein cannot produce high quality X-ray diffraction to be optimized using MPCS. Therefore, the protein assays in this study initially produced a number of crystal samples but were otherwise randomly selected. In summary, 29 proteins are optimized by MPCS.
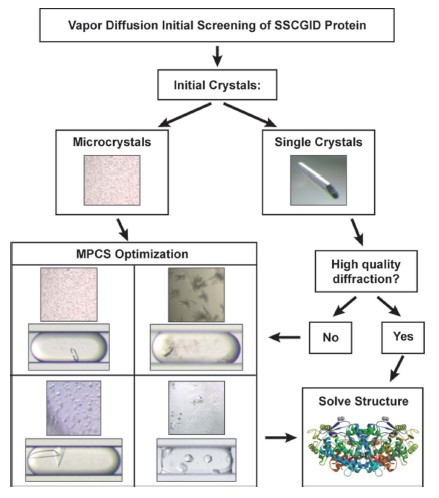
Figure 1: Flow chart of the work of this study. Purified proteins were obtained and subjected to preliminary screening by hanging drop method of meteorological diffusion experiments. If the crystal obtained initially is a single crystal and can be harvested, X-ray diffraction analysis can be performed. If the original protein crystals can produce high quality X-ray diffraction data, the protein structure can be optimized without MPCS. However, if the initial X-ray diffraction data is not good or the original protein crystals are small or not harvested, then MPCS optimization is required.
The high-grained gradient produced by the MPCS optimization experiment contains up to 400 separate crystals in 20nL of droplets called droplets. Approximately 2 μL of residual protein and 2 μL of precipitant solution (for primary screening) were combined in an MPCS CrystalCard (Fig. 2a). There is a slight difference in the concentration of each droplet formation compared to the previous or subsequent droplets. This can be done by kinetics controlling the flow rate of the droplet formation solution. Computer flow control produces a variety of potential gradients. Simply put, only two gradients were applied to the study. These two types are shown in Figures 2(b) and 2(c). The goal of both MPCS optimization types is to carefully interrogate the initial impact around the narrow region of the crystalline phase space. Type 1 optimization maintains the protein concentration of all droplets while changing the concentration of the precipitant. Type 2 is optimized for different protein and precipitant concentrations, and the other side is used to detect different ratios of protein and precipitant. The optimization experiment was completed and stored on a CrystalCard with a humidity of 100% for crystal growth. Crystalline droplets stored in CrystalCard at 100% humidity can be stored for 6 months. In addition, although no further studies were conducted in this study - droplets were automatically dehydrated by controlling the reservoir humidity during crystal growth on the CrystalCard. After crystal growth, it was used for X-ray diffraction analysis by stripping off a thin plastic adhesive layer (Fig. 2d) and harvesting protein crystals directly from the microtubes (Fig. 2e).
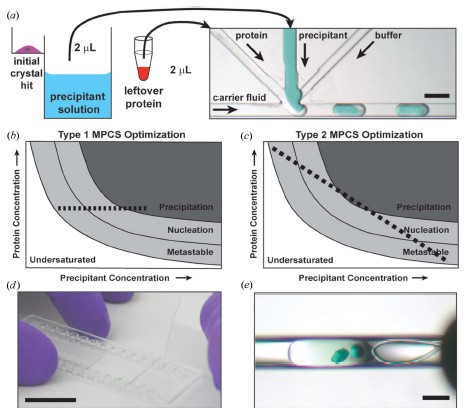
Figure 2: (a) Approximately 2 μL of residual protein and 2 μL of precipitant solution remaining in the initial experiment (left) were used for the optimization experiment of MPCS CrystalCard (right). On the CrystalCard, the aqueous solution (protein, precipitant and buffer) combines and spontaneously forms separate droplets through an inert, non-mixable transfer fluid. As a result, the droplets will fill the microtubes and then be used in separate crystallization experiments. Scale bar = 400 μm. (b), (c) Protein phase diagram showing how the crystalline phase space is formed in the MPCS optimization. In the type 1 MPCS optimization, (b) the protein concentration remains unchanged and the precipitant forms a series of concentration gradients. In type 2 MPCS optimization, (c) protein concentration begins with a high subsequent slow decrease and the precipitant concentration initially decreases and then slowly increases, resulting in a series of droplets of protein and precipitant kinetic gradients. (d) A Crystal Card image in which the adhesive layer was peeled off to expose the crystal. Scale ruler = 1 inch ≈ 2.54 cm. (e) Protein crystallization was harvested from the CrystalCard using a 0.2 mm cryo-loop. Scale bar = 200 μm.
3. Materials and methods
The plastic CrystalCard is made of an olefin copolymer. Each CrystalCard has two separate microtubes with a volume of approximately 10 μL. An optimized experiment can be performed for each microtube. The formation of droplets in a CrystalCard requires a surface with a low surface energy. This ensures that the transfer fluid (FC-40) preferentially wets the fine tube wall. To prepare the surface of the microtube formed by the droplets, a solution of Cytonix PFC 502AFA was applied to the inside of the microtube. Using a coating, the CrystalCard is filled through the Cytonix 502AFA inlet and can be stored for 0.5 to 1 hour at room temperature. The 502 AFA solution was removed from the CrystalCard by vacuum followed by curing at 333 - 343 K for 1 hour.
The CrystalCard has four liquid inlets, followed by transfer solution (1), protein (2), precipitant (3) and buffer (4). The buffer, protein and precipitant channels are combined into a 3 + 1 mixer where the aqueous solution is combined and split into individual droplets of inert and immiscible fluid carrier. The syringe and Teflon tube are backfilled by the transfer fluid and the required aqueous solution is drawn from the end of the Teflon sleeve. The CrystalCard connection is completed through a Teflon tube and a polypropylene connector to form a sealed CrystalCard port. The experimental composition of the liquid was placed in a Teflon tube with a syringe pump and was transferred to CrystalCard to some extent by the previously used MicroPlugger pump control software (Gerdts et al., 2008). The liquid configuration on the CrystalCard is shown in Figure 2.
The flow rate of the aqueous solution can be varied so that a series of gradient droplets can be produced. In this study, we used two types of gradients for the first time (Table 1 and Figure 3). In the type 1 gradient, the protein was delivered at a constant rate (2 μL min-1) although the precipitant and buffer formed a linear gradient (the total precipitant and buffer flow remained constant at 2 μL min-1). For most type 1 gradients, the precipitant flow rate is initially set to 2 μL min-1, then the flow rate is slowly reduced and the buffer is slowly increased at the same rate. Therefore, in all Type 1 experiments, the protein concentration remained constant only changing the concentration of the precipitant (Table 1). In Gradient Type 2, a dynamic gradient is created between the protein and the precipitant. In Gradient 2, the protein flow was slowly reduced, the precipitant flow was slowly increased, and the buffer flow was kept constant (Table 1). In general, a type 1 gradient first produces a protein/precipitant combination, and if there is no crystal screening, a type 2 gradient is usually produced immediately.
Table 1: Type 1 and Type 2 MPCS Gradient Flow Meters (μL min-1) used in this study
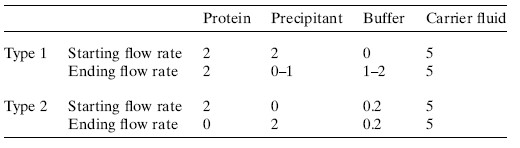
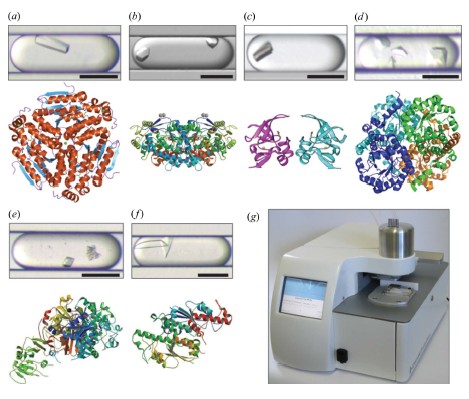
Figure 3: High quality data set (2.5 A or better) obtained by MPCS optimization of crystals and/or new protein structure. The corresponding protein structure map of the droplets (see Table 2 for data collection and statistics). All scales = 200 μm. (a) Enoyl-CoA hydratase obtained from Mycobacterium tuberculosis (1.8 A °; PDB code 3h81); (b) aldehyde dehydrogenase obtained from Bartonella henselae (2.1 A °; PDB code 3i44; optimized by sitting drop method) The resulting 2.0 A ° coded 3i44 structure has been deposited into the PDB database, the 2.1 A ° resolution data set was optimized by MPCS crystallization; (c) the methionine-R-sulfoxide reductase obtained from Burkholderia pseudomallei ( 1.7 A ° ; PDB code 3cxk); (d) methyl isocitrate lyase (2.9 A ° ; PDB code 3eoo) obtained in Brucella melitensis; (e) dihydrofolate reductase/thymidine obtained in Babesia bovis Acid synthase (2.5 A ° ; PDB code 3i3r); (f) tRNA guanine-n1-transmethylase (2.5 A °; PDB code 3ief) obtained from Bartonella henselae; (g) commercialized MPCS Plug Maker image. Left: User interface and touch screen for CrystalCard imaging. Right: The instrument sample stage is used to load CrystalCard and crystallized samples.
Precipitants used in this study can be obtained from Emerald Biosystems (Wizard I, Wizard II, Wizard III, JCSG+ and Precipitant Synergy) or Hampton Research (Crystal Screen HT and Index HT). User-specified Wizard I and Wizard II are also available. The main precipitant concentrations of Wizard I and Wizard II are increased by 50-100%.
Crystals directly extracted from CrystalCard can be directly used for downstream X-ray diffraction analysis (Fig. 2d). The 100 μm thick adhesive layer was peeled off to expose the desired crystals for harvesting. Approximately 1 microliter of the previously prepared cryoprotectant is typically drawn onto the desired crystals. The crystals were then removed from the microtubes using conventional nylon cold ring vitrification (about 2 mm diameter, Hampton Research) and stored in liquid nitrogen for X-ray analysis. In individual cases, crystals were found in the 100 μm tie layer. In this case, the crystals are covered by the cryoprotectant and harvested from the top of the plastic layer.
4. Results
The MPCS gradient has a high success rate in optimizing the number of meteorological diffusion crystal samples. Of the 29 proteins optimized by MPCS, 28 (93%) were crystallized using MPCS optimization. Many of the proteins used in this study used more than one precipitant solution in the initial screening. In summary, 120 different protein/precipitant crystal combinations were produced in meteorological diffusion methods. Among the 120 combinations, the success rate of 90 (75%) crystal-crystals produced in the MPCS optimization experiment is very high, many of the original crystals are not single crystals, but the crystallites or precipitates look like crystals (Figure 1 ). In addition, the MPCS experimental volume was significantly reduced (50-100 times) while the crystals obtained from the meteorological diffusion method were converted into batch-under-oil-style crystals. Again, 10 precipitant solutions that did not crystallize in the meteorological diffusion method were used in MPCS optimization experiments to produce crystals. These special precipitants were tested with MPCS (although they could not be crystallized by meteorological diffusion methods) because they have similar chemical compositions to other precipitant solutions that produce crystals in meteorological diffusion experiments. In total, 17 precipitant solutions were tested using this method, and ten of them produced crystals (59%). This suggests that the use of MPCS to optimize each precipitant in standard crystal screening may result in many specific crystal collections that may fail in a single meteorological diffusion test—with batch-under-oil-style crystallization Meteorological spread is consistent with previously reported data (Baldock et al., 1996; D'Arcy et al., 2003). More than 90% of the precipitant solution was detected by meteorological diffusion test using MPCS, which indicates that the use of MPCS has the potential to discover new crystal collection numbers. Various precipitant solutions were used in this study, including various salt solutions, low pH (4.5) to high pH (10.5) solutions, high viscosity polyethylene glycol solutions and organics such as isopropanol and 2-methyl -2,4-pentanediol.
5. Discussion
The goal of MPCS optimization is to obtain protein structure from the initial pass (1) when the hanging drop method does not produce a single crystal or (2) to improve the diffraction quality of the single crystal produced by the hanging drop method. The 29 proteins in this study were optimized using MPCS to obtain 6 new protein structures and submitted to PDB with a success rate of 21% (Fig. 3a-3f; crystallization optimization data are shown in Table 2). This yield is not inferior to published reduction methylation (Kim et al., 2008) and limited proteolysis (Dong et al., 2007). In both cases, high-quality diffraction can also be obtained by crystal growth in a downstream suspended-drip meteorological diffusion test (in one case, the MPCS crystal has a slightly higher resolution for X-ray diffraction, and the other is MPCS). The diffraction resolution of the crystal is slightly lower). ATCG3D will continue to apply MPCS throughout the study, resulting in the commercial MCPS Plug Maker (Figure 3g). This study shows that MPCS technology is a successful method to optimize protein crystallization in order to obtain high quality X-ray diffraction results. The future direction of this technology is to develop high protein samples in the lipidic cubic phase crystallization process (Li et al., 2009) and the hybrid method (Li et al., 2006) (sparse matrix + gradient screening). The initial screening of the flux makes it possible to automate the MPCS Plug Maker.
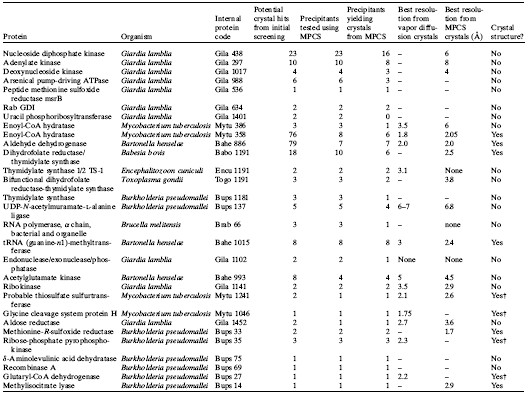
Table 2: Crystallization optimization data
Structure obtained through downstream research after completion of the study
references:
[1] Baldock, P., Mills, V. & Shaw Stewart, P. (1996). J. Cryst. Growth, 168, 170–174.
[2] Cherezov, V., Hanson, MA, Griffith, MT, Hilgart, MC, Sanishvili, R., Nagarajan, V., Stepanov, S., Fischetti, RF, Kuhn, P. & Stevens, RC (2009) JR Soc. Interface, 6 Suppl. 5, S587–597.
[3] Cherezov, V., Liu, J., Griffith, M., Hanson, MA & Stevens, RC (2008). Cryst. Growth Des. 8, 4307–4315.
[4] D'Arcy, A., Mac Sweeney, A., Stihle, M. & Haber, A. (2003). Acta Cryst. D59, 396–399.
[5] Dhouib, K., Khan Malek, C., Pfleging, W., Gauthier-Manuel, B., Duffait, R., Thuillier, G., Ferrigno, R., Jacquamet, L., Ohana, J. , Ferrer, JL, Teheobald-Dietrich, A., Giege, R., Lorber, B. & Sauter, C. (2009). Lab-On-A-Chip, 9, 1412–1421.
[6] Dong, A. et al. (2007). Nat. Methods, 4, 1019–1021.
[7] Fox, BG, Goulding, C., Malkowski, MG, Stewart, L. & Deacon, A. (2008). Nat. Methods, 5, 129–132.
[8] Gerdts, CJ, Elliott, M., Lovell, S., Mixon, MB, Napuli, AJ, Staker, BL, Nollert, P. & Stewart, L. (2008). Acta Cryst. D64, 1116–1122 .
[9] Gerdts, CJ, Tereshko, V., Yadav, MK, Dementieva, I., Collart, F., Joachimiak, A., Stevens, RC, Kuhn, P., Kossiakoff, A. & Ismagilov, RF (2006) ). Angew. Chem. Int. Ed. 45, 8156–8160.
[10] Hansen, CL & Quake, SR (2003). Curr. Opin. Struct. Biol. 13, 538–544.
[11] Hansen, CL, Skordalakes, E., Berger, JM & Quake, SR (2002). Proc. Natl Acad. Sci. USA, 99, 16531–16536.
[12] Kim, Y. et al. (2008). Nat. Methods, 5, 853–854.
[13] Li, L., Du, W. & Ismagilov, RF (2010). J. Am. Chem. Soc. 132, 112–119. Li, L., Fu, Q., Kors, CA, Stewart, L., Nollert, P., Laible, PD & Ismagilov, RF (2009). Microfluidics Nanofluidics, 8, 789–798.
[14] Li, L., Mustafi, D., Fu, Q., Tereshko, V., Chen, DL, Tice, JD & Ismagilov, RF (2006). Proc. Natl Acad. Sci. USA, 103, 19243 –19248.
[15] Ng, JD, Clark, PJ, Stevens, RC & Kuhn, P. (2008). Acta Cryst. D64, 189–197.
[16] Ng, JD, Stevens, RC & Kuhn, P. (2008). Methods Mol. Biol. 426, 363–376.
[17] Sauter, C., Dhouib, K. & Lorber, B. (2007). Cryst. Growth Des. 7, 2247–2250.
[18] Zheng, B., Gerdts, CJ & Ismagilov, RF (2005). Curr. Opin. Struct. Biol. 15, 548–555.
[19] Zheng, B., Roach, LS & Ismagilov, RF (2003). J. Am. Chem. Soc. 125, 11170–11171.
Christmas Ornaments,Clear Christmas Ornaments,Plastic Christmas Ornaments,Christmas Ball Ornament
COFCO HEBEI INTERNATIONAL TRADING CO., LTD. , https://www.cofcohb.com