Recently, Servier, Pfizer and French biotechnology company Cellectis jointly announced that the experimental new drug application (IND) for CAR-T cell therapy UCART19 has been approved by the US Food and Drug Administration (FDA). Human clinical trials can be conducted in the United States for the treatment of relapsed/refractory acute lymphoblastic leukemia (ALL) indications.
UCART19 is an allogeneic CAR-T cell therapy using Cellectis (Medical Note: On February 7, 2017, Cellectis announced that its universal CAR-T therapy UCART123 was approved by the US FDA for the first time. Developed with the proprietary allogeneic method of the FDA approved for entry into clinical trials. It is edited by Talen gene and does not need to be modified according to the patient, but will be directly derived from non-patients (non -patient) Donor T cells are engineered for treatment in multiple patients, unlike autologous methods, which engineer T cells from patients themselves, including kite pharmacy, Juno All use the autologous transplantation method. UCART19 Compared to existing T cell-based autologous products, UCART19 has the advantages of allogeneic, frozen state, "present molding" and the like to overcome the limitations of current autonomic methods.
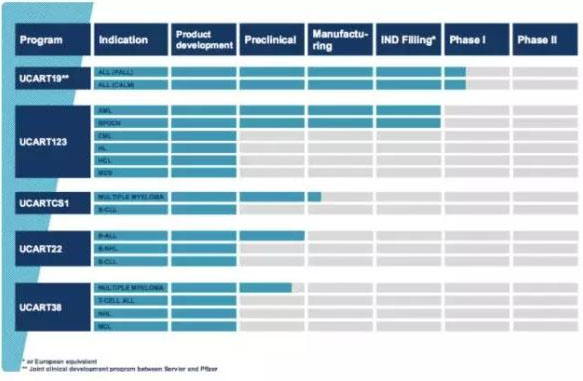
Cellectis's UCART product line
It is reported that Servier will be responsible for the Phase I clinical study CALM of UCART19. In November 2015, Servier obtained the exclusive right of UCART19 from Cellectis. The CALM clinical trial was launched in the UK in August 2016. This is an open A dose escalation study of the label was designed to assess the safety, tolerability, and anti-leukemia activity of UCART19 in patients with relapsed or refractory CD19-positive B-cell acute lymphoblastic leukemia (B-ALL). With the approval of the IND, the CALM study will be further expanded and included in multiple US cancer centers including the MD Anderson Cancer Center.

leather corner sofa,Corner Sofas,large corner sofa,corner chaise sofa
Guangzhou LoPhiDa Co.Ltd , https://www.widinlsamachine.com